Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ashida, Takashi; ; Sato, Haruo; ; Kitamura, Akira; Kawamura, Kazuhiro
JNC TN8400 99-083, 63 Pages, 1999/11
Studies on the chemical and migration behaviour of radionuclides were carried out in the Quantitative Assessment Radionuclide Migration Experimental Facility (QUALITY)for assuring the relaiability and for improving the propriety of data concerning nuclide migration used in the Second Progress Report for the geoloical disposal of high-level radioactive waste. Five studies for solubility, sorption and diffusion concerning nuclide migration were carried out. The overview of each study and the result is as follows: (1)Study on Effect of Carbonate on Np Solubility. Solubilities of Np(IV) were measured as functions of pH and carbonate concentration under reducing conditions. The obtained data could be well described by considering two hydroxo-carbonate complexes, and those stability constants were estimated and compared with the literature data. Consequently, the data obtained in this study were similar to the literature data. (2)Study on Effect of Carbonate on Np Sorption on Bentonite. Distribution coefficients (Kd) of Np(IV) on smectite were measured as a function of carbonate concentration. The obtained Kd values were approximately constant over the carbonate concentration (total carbon concentration 0.04-0.15M). The results of desorption tests by 1M KCl and HCl at the end of sorption experiments showed two different desorption behaviour; Np(IV) was well removed by HCl for the experiments in low carbonate concentration and by KCl for those in high carbonate concentration. (3)Distribution Coefficient Measurements for Cs, Pb and Cm on Rocks. Distribution Coefficients for Cs, Pb and Cm on Japanese major rocks (basalt, mudstone, sandstone, granodiorite and tuff) were measured as a function of ionic strength. The obtained Kd values were either the same orders or higher compared with data used to both fresh and saline groundwater systems in the Second Progress Report. This indicates that the Kd data used in the Second Progress Report are either proper or conservative. ...
Oda, Chie; Ikeda, Takao*; Shibata, Masahiro
JNC TN8400 99-073, 112 Pages, 1999/11
In the safety assessment for geological disposal of high-level radioactive wastes (HLW), distribution coefficients (Kd) and diffusion coefficients of radionuclides are used to estimate the migration of radionuclides in a near-field of repository.  Sn is one of the important nuclides for the safety assessment in Japan and its behavior under reopsitory conditions has not been understood. This report provides the experimental informations for the sorption of Sn on bentonite, tuff and granodiorite, and the diffusion of Sn in a compacted bentonite. The Kd values of Sn on bentonite, tuff and granodiorite were determined by the batch-type sorption experiments as l0
Sn is one of the important nuclides for the safety assessment in Japan and its behavior under reopsitory conditions has not been understood. This report provides the experimental informations for the sorption of Sn on bentonite, tuff and granodiorite, and the diffusion of Sn in a compacted bentonite. The Kd values of Sn on bentonite, tuff and granodiorite were determined by the batch-type sorption experiments as l0
 10
10 [ml/g], 10
[ml/g], 10
 10
10 [ml/g] and 10
[ml/g] and 10
 10
10 [ml/g], respectively. The sequential extraction experiments for adsorbed Sn on bentonite were also performed to investigate its desorption behavior. These experimental results indicated that the mechanisms of sorprion onto bentonite were dominated by the sorption reactions on smectite and pyrite and consisted of reversible and irreversible sorption on solid and stable fixation in solid. On the other hands, the apparent diffusion coefficients (Da) in compacted bentonite were measured by the diffusion experiments as 10
[ml/g], respectively. The sequential extraction experiments for adsorbed Sn on bentonite were also performed to investigate its desorption behavior. These experimental results indicated that the mechanisms of sorprion onto bentonite were dominated by the sorption reactions on smectite and pyrite and consisted of reversible and irreversible sorption on solid and stable fixation in solid. On the other hands, the apparent diffusion coefficients (Da) in compacted bentonite were measured by the diffusion experiments as 10 [m
[m /sec] and l0
/sec] and l0 [m
[m /sec] for dry densities of 0.4[g/cm
/sec] for dry densities of 0.4[g/cm ] and 1.0[g/cm
] and 1.0[g/cm ], respectively. Moreover, the Kd values in compacted bentonite were calculated according to the relationship with the measured Da values, and the solubilities in the porewaters of compacted bentonite were calculated by use of the calculated Kd and the obtained diffusion plofiles. It is found that the derived solubilities almost agreed with the solubiliies of amorphis SnO
], respectively. Moreover, the Kd values in compacted bentonite were calculated according to the relationship with the measured Da values, and the solubilities in the porewaters of compacted bentonite were calculated by use of the calculated Kd and the obtained diffusion plofiles. It is found that the derived solubilities almost agreed with the solubiliies of amorphis SnO reported by Amaya et al. (1997), however, the derived Kd values were lower than that measured from the batch-type sorption experiments.
reported by Amaya et al. (1997), however, the derived Kd values were lower than that measured from the batch-type sorption experiments.
Sato, Haruo
JNC TN8400 99-065, 379 Pages, 1999/10
A database for diffusivity for a data setting of effective diffusion coefficients in rock matrices in the second progress report, was developed. In this database, 3 kinds of diffusion coefficients: effective diffusion coefficient (De), apparent diffusion coefficient (Da) and free water diffusion coefficient (Do) were treated. The database, based on literatures published between 1980 and 1998, was developed considering the following points. (1)Since Japanese geological environment is focused in the second progress report, data for diffusion are collected focused on Japanese major rocks. (2)Although 22 elements are considered to be important in performance assessment for geological disposal, all elements and aquatic tracers are treated in this database development considering general purpose. (3)Since limestone, which belongs to sedimentary rock, can become one of the natural resources and is inappropriate as a host rock, it is omitted in this database development. Rock was categorized into 4 kinds of rocks; acid crystalline rock, alkaline crystalline rock, scdimentaly rock (argillaceous/tuffaceous rock) and sedimentary rock (psammitic rock/sandy stone) from the viewpoint of geology and mass transport. In addition, rocks around neutrality among crystalline rock were categorized into the alkaline crystalline rock in this database. The database is composed of sub-databases for 4 kinds of rocks. Furthermore, the sub-databases for 4 kinds of the rocks are composed of databases to individual elements, in which totally, 24 items such as species, rock name, diffusion coefficients (De, Da, Do), obtained conditions (method, porewater, pH, Eh, temperature, atmosphere, etc.), etc. are input. As a result of literature survey, for De values for acid crystalline rock, totally, 207 data for 18 elements and one tracer (hydrocarbon) have been reported and all data were for granitic rocks such as granite, granodiorite and biotitic granite. For alkaline crystallinc rock, ...
; ; Sato, Haruo; Shibata, Masahiro
JNC TN8400 99-088, 58 Pages, 1999/06
Sorption and diffusion behavior of palladium, which has been identified as one of the hazardous radionuclides in performance assessment of HLW disposal, in bentonite, granodiorite and tuff was studied in order to make reliable data set for the performance assessment. Sorption experiments of Pd on bentonite, granodiorite and tuff were conducted as functions of pH, ionic strength and liquid to solid ratio by batch method under aerobic conditions at room temperature. The distribution coefficients (K ) of Pd on these solids were almost in the range of 10
) of Pd on these solids were almost in the range of 10 to 10
to 10 m
m /kg and were in the order of bentonite
/kg and were in the order of bentonite  granodiorite
granodiorite 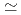 tuff. The sorption trends with change in PH, ionic strength and liquid to solid ratio are very similar between three solids. The K
tuff. The sorption trends with change in PH, ionic strength and liquid to solid ratio are very similar between three solids. The K values were the highest at pH5 and decreased with increasing pH between 5 and 11. The effect of ionic strength on K
values were the highest at pH5 and decreased with increasing pH between 5 and 11. The effect of ionic strength on K was not found in a range of 10
was not found in a range of 10 to 10
to 10 , but K
, but K values increased with increasing liquid to solid ratio. The width of variation in K
values increased with increasing liquid to solid ratio. The width of variation in K was one order of magnitude in a liquid to solid ratio of 0.1 to 1 m
was one order of magnitude in a liquid to solid ratio of 0.1 to 1 m /kg. Sorption behavior of Pd is different from that of divalent metal ions such as Ni and Co etc. and chemical analogy may be inappropriate. The dominant aqueous species of Pd in the expermental conditions studied is estimated to be neutral species, Pd(OH)
/kg. Sorption behavior of Pd is different from that of divalent metal ions such as Ni and Co etc. and chemical analogy may be inappropriate. The dominant aqueous species of Pd in the expermental conditions studied is estimated to be neutral species, Pd(OH) (aq) by the thermodynamic calculations. The K
(aq) by the thermodynamic calculations. The K values of Pd on three solids were relatively high and uncharged complexes may be more strongly sorbed. The pH dependency of K
values of Pd on three solids were relatively high and uncharged complexes may be more strongly sorbed. The pH dependency of K values suggests that Pd sorption is most likely to be occurring onto positively charged S-OH
values suggests that Pd sorption is most likely to be occurring onto positively charged S-OH type site which are progressively removed (to from SOH and SO
type site which are progressively removed (to from SOH and SO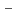 sites) at higher pH values. Diffusion behavior of Pd in bentonite was also studied by in-diffusion method as a function of dry density. The D
sites) at higher pH values. Diffusion behavior of Pd in bentonite was also studied by in-diffusion method as a function of dry density. The D values obtained based on the instantaneous planar source model were in the orders of ...
values obtained based on the instantaneous planar source model were in the orders of ...
Ishikawa, Kiyoshi*; Mezaki, Yoshihiko*; Suzuki, Hideo*; Kai, Masanori*; Watanabe, Hajime*; Fujimori, Seiji*; Ishikawa, Junichi*
JNC TJ7420 99-016, 878 Pages, 1999/06
no abstracts in English
Okubo, Seisuke*
PNC TJ1602 98-004, 87 Pages, 1998/03
no abstracts in English
 Np and
Np and  Am in sedimentary geological formation
Am in sedimentary geological formationTanaka, Tadao; Takebe, Shinichi; Ogawa, Hiromichi; Muraoka, Susumu
JAERI-Research 98-018, 20 Pages, 1998/03
no abstracts in English
Ueta, Shinzo*
PNC TJ1211 98-002, 46 Pages, 1998/02
None
Mukai, Masayuki; Takebe, Shinichi; Komiya, Tomokazu
JAERI-Research 94-014, 25 Pages, 1994/09
no abstracts in English
; Kumata, Masahiro
Butsuri Tansa, 47(3), p.161 - 172, 1994/00
no abstracts in English
 Co,
Co,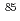 Sr and
Sr and  Cs in a porous tuff; Mechanisms and kinetics
Cs in a porous tuff; Mechanisms and kineticsC.K.Park*; S.I.Woo*; Tanaka, Tadao; Kamiyama, Hideo
Journal of Nuclear Science and Technology, 29(12), p.1184 - 1193, 1992/12
no abstracts in English
Shibutani, Tomoki; Yoshikawa, Hideki; Sato, Haruo; Yui, Mikazu; Umeki, Hiroyuki; Ishiguro, Katsuhiko
PNC TN8410 92-163, 51 Pages, 1992/09
None
Tanaka, Tadao; K.Sriyotha*; Kamiyama, Hideo
Proc. of the 3rd Int. Conf. on Nuclear Fuel Reprocessing and Waste Management: RECOD91,Vol. 2, p.1011 - 1016, 1991/00
no abstracts in English
Journal of Materials Science Letters, 9, p.841 - 844, 1990/00
Times Cited Count:1 Percentile:11.89(Materials Science, Multidisciplinary)no abstracts in English
; *; *; ; Araki, Kunio;
JAERI-M 9247, 28 Pages, 1980/12
no abstracts in English